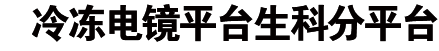Structural basis of assembly of the human T cell receptor–CD3 complex
The αβ T cell receptor (TCR), in association with the CD3γε–CD3δε–CD3ζζ signalling hexamer, is the primary determinant of T cell development and activation, and of immune responses to foreign antigens. The mechanism of assembly of the TCR–CD3 complex remains unknown. Here we report a cryo-electron microscopy structure of human TCRαβ in complex with the CD3 hexamer at 3.7 Å resolution. The structure contains the complete extracellular domains and all the transmembrane helices of TCR–CD3. The octameric TCR–CD3 complex is assembled with 1:1:1:1 stoichiometry of TCRαβ:CD3γε:CD3δε:CD3ζζ. Assembly of the extracellular domains of TCR–CD3 is mediated by the constant domains and connecting peptides of TCRαβ that pack against CD3γε–CD3δε, forming a trimer-like structure proximal to the plasma membrane. The transmembrane segment of the CD3 complex adopts a barrel-like structure formed by interaction of the two transmembrane helices of CD3ζζ with those of CD3γε and CD3δε. Insertion of the transmembrane helices of TCRαβ into the barrel-like structure via both hydrophobic and ionic interactions results in transmembrane assembly of the TCR–CD3 complex. Together, our data reveal the structural basis for TCR–CD3 complex assembly, providing clues to TCR triggering and a foundation for rational design of immunotherapies that target the complex.
https://www.nature.com/articles/s41586-019-1537-0