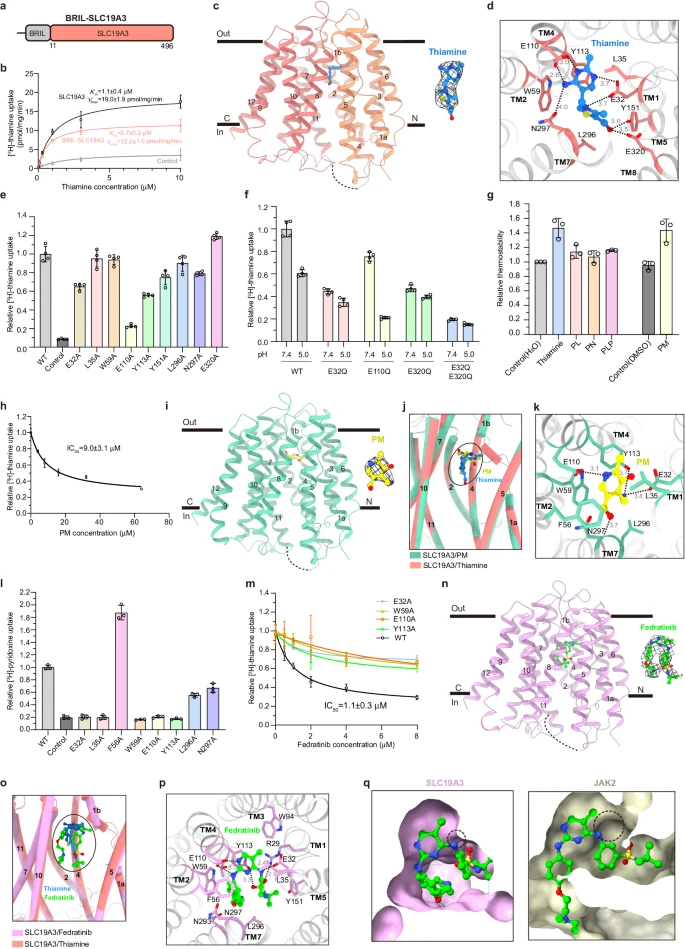
Substrate and drug recognition mechanisms of SLC19A3
Vitamins B1 and B6 are two water-soluble vitamins. Their active forms thiamine pyrophosphate and pyridoxal 5′-phosphate serve as cofactors for numerous enzymes involved in multiple biochemical reactions that are essential to maintain the composition and energy metabolism of the human body.1 Consequently, their deficiency leads to a variety of diseases, such as neurological abnormalities and cardiovascular diseases.2 Humans cannot synthesize these vitamins de novo and must obtain them from the diet. Two specific transporters, thiamine transporter 1 (ThTr1 or SLC19A2) and thiamine transporter 2 (ThTr2 or SLC19A3), have been identified to be the major transmembrane transporters that are involved in the uptake of both vitamins thus far.3,4,5,6 Interestingly, the clinical antineoplastic drug fedratinib, a Janus kinase 2 (JAK2) inhibitor used in treating myelofibrosis, induces Wernicke’s-like encephalopathy in some myelofibrosis patients due to thiamine scarcity.7,8 This is caused by its off-target inhibitory activity against both SLC19A2 and SLC19A3.9 Here, we report the cryo-electron microscopy (cryo-EM) structures of human SLC19A3 in complex with vitamins B1, B6, or fedratinib. Remarkably, all these compounds bind to the same site on SLC19A3 via a closely related structural element. Mutagenesis studies further revealed the critical residues of SLC19A3 for substrate and drug recognition.