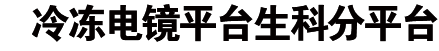Structure of the mitochondrial TIM22 complex from yeast
Mitochondria have more than 1000 proteins, most of which are encoded by nuclear DNA and imported from the cytosol.1 A large group of multi-spanning membrane proteins, the solute carrier proteins, are imported from the cytosol by the translocase of the outer membrane (TOM) and translocated into the inner membrane (IM) by the TIM22 complex.2 The TIM22 complex in Saccharomyces cerevisiae (S. cerevisiae) has seven subunits: Tim22, Tim18, Tim54, Sdh3, Tim9, Tim10, and Tim12. Tim22 is the core translocase subunit. The three small Tim proteins, Tim9, Tim10, and Tim12, are homologous to each other. Tim9 and Tim10 form hexameric chaperones in the intermembrane space (IMS) to deliver the polypeptide substrates from the TOM to the TIM22.3 Tim9 and Tim10 become part of the TIM22 complex with the help of Tim12.4 Tim54 likely interacts with Tim9-Tim10-Tim12 in the complex.5 Sdh3 is a membrane component of respiratory complex II (succinate dehydrogenase, SDH). Tim18 is homologous to Sdh4, the partner of Sdh3 in respiratory complex II. The functions of Sdh3 and Tim18 in the TIM22 complex are less clear. Previous studies have suggested that driven by the membrane potential, the TIM22 complex might function as a twin-pore translocase.6 However, it is poorly understood how the subunits of TIM22 assemble and accomplish protein translocation due to lack of high-resolution structural information.
https://www.nature.com/articles/s41422-020-00399-0