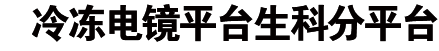Cryo-EM structure of full-length α-synuclein amyloid fibril with Parkinson’s disease familial A53T mutation
α-Synuclein (α-Syn) forms amyloid fibrils accumulating in Lewy bodies (LB) and Lewy neuritis (LN), which is a common histological hallmark of Parkinson’s disease (PD) and other synucleinopathies.1 Moreover, α-syn amyloid fibrils spread in the patient’s brain via cell-to-cell transmission, which accounts for the disease progression.2 Several single amino-acid mutations of α-syn, including A53T, E46K, H50Q, G51D, A30P and A53E, have been identified from familial PD patients, which are causative to the early-onset pathology with different clinical symptoms.3 Among them, A53T is the first hereditary mutation of α-syn discovered in Italian and Greek families with autosomal dominant and early-onset PD.4 Till now, A53T represents the most commonly reported cases of familial PD involving more than 10 families of Greek, Korean, Swedish and Chinese origin.3 A53T mutation promotes α-syn fibril formation in vitro, and exhibits exacerbated PD-like pathology both in cellular and animal models.5,6 Previous structural studies on the wild-type (WT) α-syn fibril reveal that A53 is one of the key residues in the interface of α-syn protofilaments.7,8,9 However, it remains unclear how the A53T mutation may alter the fibril structure and exacerbate α-syn pathology.
https://www.nature.com/articles/s41422-020-0299-4