HIV-1 Env trimers asymmetrically engage CD4 receptors in membranes
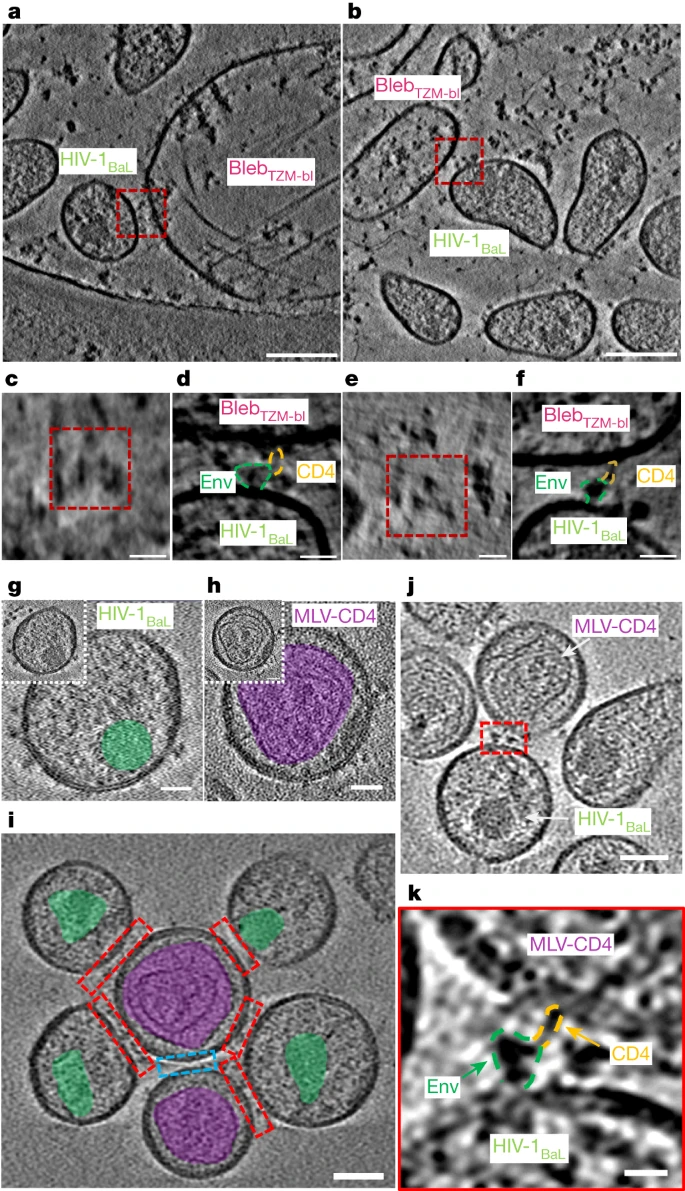
Human immunodeficiency virus 1 (HIV-1) infection is initiated by binding of the viral envelope glycoprotein (Env) to the cell-surface receptor CD41,2,3,4. Although high-resolution structures of Env in a complex with the soluble domains of CD4 have been determined, the binding process is less understood in native membranes5,6,7,8,9,10,11,12,13. Here we used cryo-electron tomography to monitor Env–CD4 interactions at the membrane–membrane interfaces formed between HIV-1 and CD4-presenting virus-like particles. Env–CD4 complexes organized into clusters and rings, bringing the opposing membranes closer together. Env–CD4 clustering was dependent on capsid maturation. Subtomogram averaging and classification revealed that Env bound to one, two and finally three CD4 molecules, after which Env adopted an open state. Our data indicate that asymmetric HIV-1 Env trimers bound to one and two CD4 molecules are detectable intermediates during virus binding to host cell membranes, which probably has consequences for antibody-mediated immune responses and vaccine immunogen design.
https://www.nature.com/articles/s41586-023-06762-6