Structural insights into the mechanism of pancreatic KATP channel regulation by nucleotides
日期:2022-05-19 | 点击量:
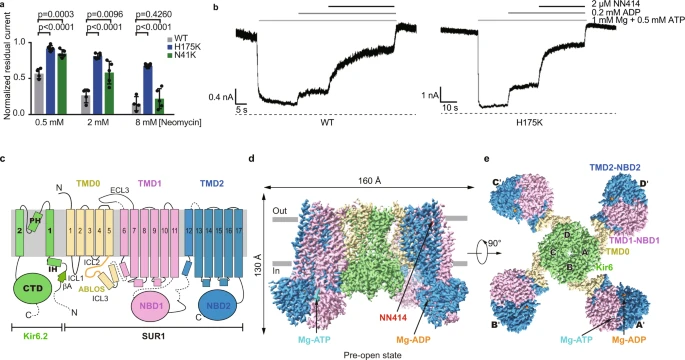
ATP-sensitive potassium channels (KATP) are metabolic sensors that convert the intracellular ATP/ADP ratio to the excitability of cells. They are involved in many physiological processes and implicated in several human diseases. Here we present the cryo-EM structures of the pancreatic KATP channel in both the closed state and the pre-open state, resolved in the same sample. We observe the binding of nucleotides at the inhibitory sites of the Kir6.2 channel in the closed but not in the pre-open state. Structural comparisons reveal the mechanism for ATP inhibition and Mg-ADP activation, two fundamental properties of KATP channels.
https://www.nature.com/articles/s41467-022-30430-4