Structural insights into VAChT neurotransmitter recognition and inhibition
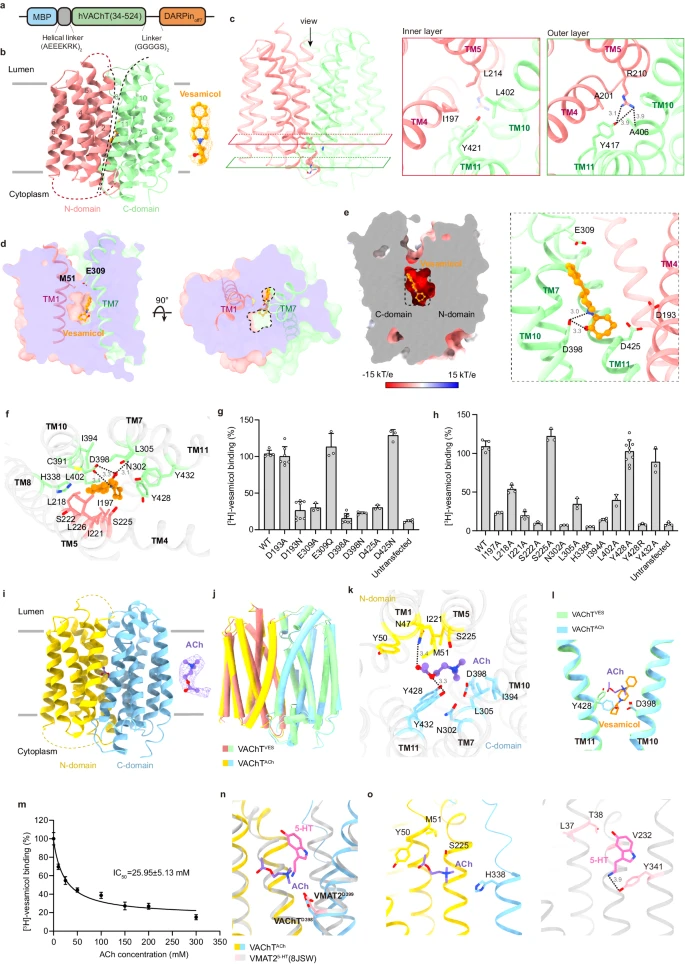
Acetylcholine (ACh) is an excitatory neurotransmitter with a wide variety of functions. In the central nervous system, ACh helps regulate memory, motivation, arousal, and attention. ACh controls muscle contraction, blood pressure, intestinal peristalsis, and glandular secretion in the peripheral nervous system. Decreased ACh signaling is strongly related to multiple pathological conditions, such as Alzheimer’s disease (AD), Lambert–Eaton myasthenic syndrome, and myasthenia gravis.1 ACh is synthesized in the cytosol, and its active transport into synaptic vesicles is mediated by vesicular acetylcholine transporter (VAChT or SLC18A3).2 Remarkably, VAChT plays a vital role in ACh neurotransmission. The vesicular accumulation of ACh is necessary for an efficient secretion of neurotransmitters in response to upstream Ca2+ signals. Knockout or knockdown of VAChT in mice severely impairs neuromuscular and cognitive functions.3,4 Furthermore, genetic variants in VAChT can cause a congenital myasthenic syndrome in humans.5 Like other vesicular neurotransmitter transporters, VAChT is a proton antiporter that pumps ACh into synaptic vesicles through the coupled efflux of protons.6 Currently, vesamicol is the best-characterized inhibitor of VAChT with nanomolar affinity.7 Vesamicol is used for functional studies on VAChT and as a specific marker of the cholinergic system. Given that AD is typically associated with the degeneration of cholinergic neurons, radiolabeled analogs of vesamicol have the potential for application in the early diagnosis of AD.8 To investigate the inhibitory mechanism of vesamicol and guide subsequent efforts to develop drugs, we determined the cryo-electron microscopy (cryo-EM) structure of human VAChT in complex with vesamicol or its native substrate ACh. In both structures, VAChT adopts a lumen-facing conformation. Although vesamicol and ACh share a conserved binding mode, they each occupy a unique pocket. Our mutagenesis studies further pinpointed the critical residues of VAChT essential for vesamicol recognition. Overall, our work provides a structural framework for clarifying how VAChT recognizes its substrates and inhibitors.
原文链接:https://www.nature.com/articles/s41422-024-00986-5